S.S. Razavi-Tousi* , J.A. Szpunar*
* Department of Mechanical Engineering, University of Saskatchewan, S7N 5A9 Saskatoon, Saskatchewan, Canada
Aluminum-water reaction is known as a candidate for production of hydrogen; this reaction produces 1.24 litter of hydrogen per 1 gr of Al. The major difficulty for this reaction to occur is formation a hydroxide layer on the surface of Al that slows the reaction process and stops it before Al is consumed. Extensive investigations have been done to promote this reaction, most of them considered high energy ball milling of Al powder to modify size, microstructure and activation of Al powder.
Scanning electron microscopy (SEM) serves as a powerful method to investigate effect of ball milling on size and morphology of Al particles and consequently on the reaction kinetics. However, the capability of SEM in observing the internal structure of the ball milled Al particles remained rather obscure so far. This research attempts to establish importance of SEM cross sectional analyses for understanding the Al-water reaction mechanism, deformation mechanism of Al particles during ball milling and effect of ball milling on the reaction kinetics.
We mounted the ball milled Al particles before and after reaction in a carbon resin powder. The mounted particles were ground to a mirror like finish and the cross section of the particles were studied by a Hitachi SU6600 SEM. We investigated particles by secondary electron (SE), backscattered electrons (BSE), energy dispersive spectroscopy (EDS) and electron backscattered diffraction (EBSD) analyses.
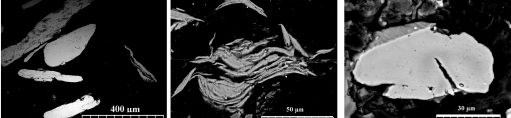
Structural evolution of Al particles during ball milling explains effect of milling duration on hydrogen generation rate. Fig. 1 shows SEM images of cross section of the ball milled particles. One observes that not only size of the particles was modified with the milling duration, but also the internal microstructure of the particles significantly changes during ball milling. This internal spaces in the laminated microstructure of 7 h milled particles (Fig. 1(b)) increases the specific surface area of the particles and explains the highest hydrogen generation rate for 7 h milled sample.
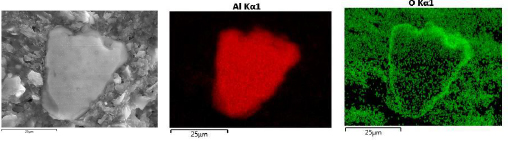
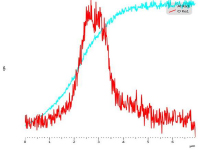
The cross sectional SEM studies also shed light on the mechanism of the reaction. As an example, Fig. 2 shows EDS of the cross section of 19 h milled sample reacted for 22 minutes with water. The aluminum hydroxide phase forms a shell around the Al core. This observation approves that reaction of Al particles and water proceeds through forming a core-shell structure. Fig. 3 shows the EDS line scan analysis of an Al particle reacted for 22 min with water. The intensity of O and Al changes along the thickness of the hydroxide layer. The overall intensity of both elements is lower for the outer layer of hydroxide film compared to the inner layer adjacent to Al core. This gradient in intensity is explained by the increasing amount of pores near the outer layer of the hydroxide film. Moreover the ratio of intensity of Al to O is changing along the thickness of the film, implying that the composition of the film is not the same in all points.
In addition to the mentioned findings, EBSD results from cross section of the Al particles milled for different times showed that formation of low-angle grain boundaries and their transformation to high angle is the main mechanism for grain refinement during ball milling. Accordingly, these findings approve the outstanding potential of cross sectional analyses of particles with SEM for a better understanding of particulate processes.
1. Soler, L., J. Macanas, J Power Sources, 169 (2007) 144.
2. S.S. Razavi-Tousi, J.A. Szpunar, Int J Hydrogen Energ, 38 (2013) 795